Classification & Registration with EDE/ MOHAP
If you’re involved in the healthcare or pharmaceutical sector, compliance with UAE health authorities is vital, nothing moves without MOHAP approvals. Nextmove assists in MOHAP registration, product classification, and licensing to ensure your healthcare products meet UAE standards.
Nextmove offers a dedicated service for MOHAP product registration and classification—designed to save you weeks of trial, error, and missed approvals.
MOHAP Compliance
Full-Service Support
Wide Product Range
Achieving Your Vision
Planning for retirement is essential to your long-term financial well-being. At Finovate, our experienced team collaborates with you to identify your retirement goals and crafts a tailored, comprehensive strategy to help you achieve them with confidence.
What we do

Import Clearance Support
We assist in obtaining MOHAP import permits and ensure hassle-free customs clearance for medical devices and pharmaceuticals, helping you comply with UAE regulations and avoid shipment delays.
Medical
Device
Classification
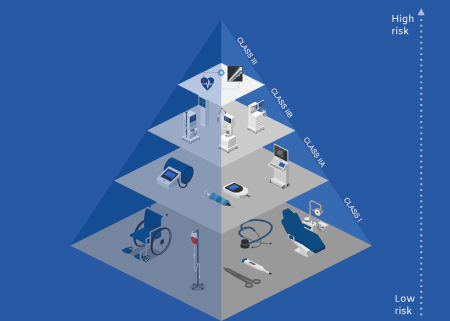
We provide expert guidance on classifying medical devices according to UAE Ministry of Health and Prevention (MOHAP) standards. Proper classification is essential for regulatory approval, ensuring compliance and smooth market entry for your products.
Medical
Device
Registration
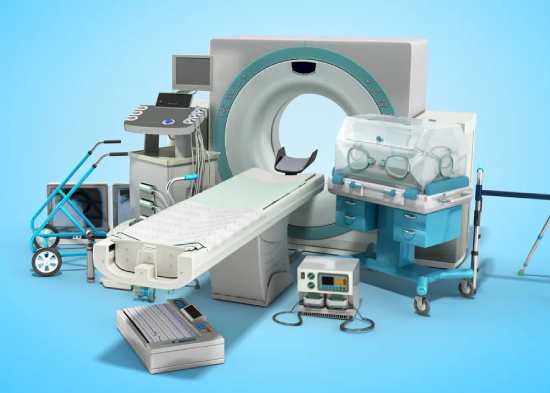
We support manufacturers, importers, and distributors in registering medical devices with UAE’s Ministry of Health and Prevention (MOHAP), ensuring full regulatory compliance for legal distribution across the UAE healthcare market.

Authorized Representative Services
We act as your local authorized representative in the UAE to ensure your medical devices meet MOHAP regulatory requirements, enabling smooth product registration, market access, and post-market communication with health authorities.
See What Our Clients Are Saying
“I hired Finovate for a small project & was very happy. He not only answered all my questions, but he didn’t treat me like a “small project”.
I was very satisfied & would recommend.”
“Finovate has been instrumental in our growth. Their team took the time to truly understand our needs and helped us eliminate inefficiencies.”
“Partnering with Finovate was a game-changer for us. They took the time to understand our challenges and helped us streamline our operations for success.”




